About Oxytocin
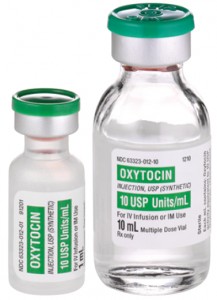
Photo courtesy of PharmPsych
Uterotonics, including misoprostol, are the most effective medicines to prevent postpartum hemorrhage, which causes one out of every four maternal deaths.
Globally, more than eight million of the 136 million women giving birth each year suffer from excessive bleeding after childbirth. This condition—medically referred to as postpartum hemorrhage (PPH)—causes one out of every four maternal deaths that occur annually and accounts for more maternal deaths than any other individual cause[1]. Deaths due to PPH disproportionately affect women in low-resource countries.
Commodity Introduction
Uterotonics are the most effective medicines to prevent postpartum hemorrhage[3]. The World Health Organization (WHO) recommends using uterotonics such as oxytocin and misoprostol during the third stage of labor to prevent PPH. Oxytocin is secreted naturally by the body during later pregnancy, labor, and when the mother breastfeeds. Synthetic forms of oxytocin are found in brand-name products as well as in generic form. In moderate doses, oxytocin produces slow, generalized contractions of the muscles in the uterus with full relaxation in between. When used for postpartum hemorrhage, oxytocin takes effect sooner than most other uterotonic drugs, including misoprostol.[4]
Commodity Context
Uterotonic medicines are needed at every level of the health care system where deliveries occur, from urban hospitals to rural clinics[5]. If uterotonic medicines, including oxytocin, were available to all women giving birth over a ten year period (2006-2016), it is projected that 41 million postpartum hemorrhage cases could be prevented and 1.4 million lives saved.[3] Excessive bleeding after childbirth can be prevented and significantly reduced with expanded availability of maternal health medicines.
Products Available
Oxytocin is most often available in 1ml glass vials, containing either 5 International Units (IU) or 10 IU. It is administered by injection into the woman’s vein or muscle.[4] Doses range between 10 IU for prevention of postpartum hemorrhage and up to 40 IU for treatment. Oxytocin has been tested in a pre-loaded and auto-disabled injection device, Uniject™, which facilitates administration, appropriate dosing, and task shifting. The medicine costs roughly US$0.18 for 10 IU (supplier median price) and is produced by more than 100 manufacturers globally.[6]
Product Efficacy
Oxytocin is recommended by WHO as the first line treatment for PPH, and is the most commonly used drug for this purpose. Oxytocin takes effect within 2-3 minutes after injection, compared to 3-5 minutes with orally administered misoprostol. However, its effect lasts about 15-30 minutes while effect of misoprostol lasts for about 75 minutes.[4]
Common Barriers
One of the major drawbacks of oxytocin is that it is temperature sensitive, and loses effectiveness after three months of being stored at temperatures higher than 30 degrees Celsius (86 degrees Fahrenheit).[4] While some manufacturers’ studies indicate that oxytocin can be stored at room temperature, the ambient temperature in tropical countries is often higher for extended periods of time.
In addition to supply-side issues such as stock-outs, there are a number of social and behavioral barriers that may also hinder the uptake of this commodity by health care providers. Health workers lack knowledge of oxytocin due to lack of access to and evaluation of up-to-date information, as well as inadequate training in the management of PPH. At the administrative level, the lack of leadership, commitment and explicit guidelines on PPH prevention and treatment (and oxytocin use specifically) leaves health workers without clear guidance on standard protocols.
For more information on oxytocin, please visit the UN Every Woman Every Child initiative webpage.
References
- Carroli, Guillermo, Cuesta, Cristina, Abalos, Edgardo, & Gulmezoglu, A Metin. (2008). Epidemiology of postpartum haemorrhage: a systematic review. Best Practice & Research Clinical Obstetrics & Gynaecology, 22(6), 999-1012.
- Children, UN Commission on Life Saving Commodities for Women and. (2012). Comissioner's Report.
- Seligman, Barbara; Liu, Xingzhu (2006). Economic Assessment of Interventions for Reducing Postpartum Hemorrhage in Developing countries: Abt Associates Inc.
- PATH. (2008). Uterotonic Drugs for the Prevention and Treatment of Postpartum Hemorrhage [factsheet]. Seattle.
- JHPIEGO, USAID;. (2012). Rapid Landscape Analysis of technologies for postpartum hemorrhage. Conducted by JHPIEGO/Accelovate for USAID at the Technologies for Health Consultative Meeting - MNCH Pathways.
- WHO, MSH;. (2010). International Drug Price Indicator Guide.

 This website is made possible by the support of the American People through the
This website is made possible by the support of the American People through the