About Female Condoms
Biologically, women are more vulnerable to HIV infection than men, and in most parts of the world women are less able than men to decide how and/or with whom they have sex. Wealth inequality, economic dependence on male partners, educational disadvantage, and norms about decision-making in relationships all increase women’s vulnerability, with younger and poorer women worse affected. Globally, approximately half of the 35.3 million people living with HIV are women, but in the generalized epidemics of sub-Saharan Africa, women account for 57% of people living with HIV[1]
Source: nih.gov
Commodity Introduction
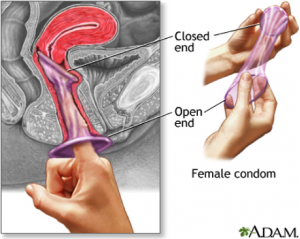
The female condom is a condom made of a soft, thin material that fits inside a woman’s vagina. Like the male condom, it is a barrier contraceptive made of latex or polyurethane, keeping the penis and sperm from contact with the cervix and vagina. But unlike the male condom, it also covers parts of the external female genitalia, ensuring that the condom stays in place, as well as protecting the female from external STIs. Current models on the market have a flexible ring, sponge, or capsule containing foam shapes at the closed end of the condom, enabling insertion of the device and helping to keep the condom in place during sex[2]. While a range of contraceptives protect against unintended pregnancies, only condoms—male and female—provide dual protection by stopping HIV transmission and preventing unintended pregnancies. When used consistently and correctly, condoms are highly effective at preventing sexually transmitted infections (STIs), including HIV. An advantage of all female condoms is that men do not need an erection to use it; therefore the woman can insert the female condom during foreplay, and the man does not need to withdraw immediately after ejaculation. It is currently the only technology that gives women and adolescent girls greater control over protecting themselves from HIV, other STIs and unintended pregnancy. The female condom does not require a prescription and has no known side effects. It can be stored and distributed easily, and can be used by women of all ages[3].
Commodity Context
Although it has yet to be seen whether female condom programming has the potential to challenge societal norms and shift sexual power dynamics, the female condom can undoubtedly play a unique role in: (1) preventing new HIV infections among women of reproductive age; and (2) helping women living with HIV avoid unintended pregnancies. Approaches that optimize the female condom’s contribution to global and national health objectives must be fully explored, and more empirically driven promotion and evaluation carried out to maximize impact and cost efficiency.

Source: Examiner
Products Available
Although there are now multiple female condom products under development, only two are WHO approved and available for bulk procurement: the FC2 and Cupid. The main female condom that has been distributed to date is the Female Health Company’s FC1/FC2. Other condoms such as the Woman’s Condom, the Phoenurse, the HLL Female Condom, the Origami female condom and the VA worn-of-women (VA w.o.w.) condom are still under development or undergoing clinical trial. Selected products are described briefly[4].
|
Product |
Illustration |
Feature |
Manufacturer |
Regulation |
|---|---|---|---|---|
|
FC2 |  | Flexible circular inner ring to insert the device and keep it in place during use, and a flexible circular outer ring to protect the genital area.Nitrile material conducts heat. | Manufactured by the Female Health Company; Distributed in 130 countries | CE marking; WHO pre-qualified 2007, renewed 2012 |
|
Cupid | 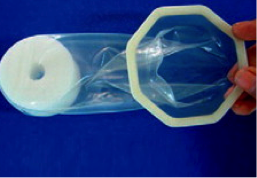 | Has an octagonal outer frame and is inserted using a medical-grade sponge which also holds the condom in place during use. Pre-lubricated with silicone oil and is the only scented female condom. | Manufactured by Cupid Ltd, India; Distributed in 7 countries | CE marking; WHO pre-qualified 2012 |
|
Woman's Condom |
| Has a soft, rounded insertion capsule which dissolves quickly after insertion in the vagina. Four foam shapes on the outside of the condom ensure internal stability. | Manufactured by Shanghai Dahua Company, China; Limited distribution | CE marking; Under review for WHO pre-qualification |
|
Phoenurse |
| The body is dumbbell-shaped, with a flexible circular inner and outer ring. Marketed with an insertion stick which attaches to the inner ring for optional use during insertion into the vagina. | Manufactured by Tianjin Condombao Medical Polyurethane Tech., China. | CE marking |
Products Efficacy
In 2011-12, the Universal Access to Female Condom (UAFC) Joint Programme supported a comparative functionality study at sites in China and South Africa of four female condoms: FC2, Cupid, the Woman’s Condom (WC), and the VA w.o.w. Total failure ranged from 3%-4.5%[5] . Programmatically, a vitally important dimension of female condom efficacy is the extent to which adding female condoms to the method mix increases the total number of protected sex acts; evaluations of female condom promotion to family planning clinic clients in California[6] and sex workers in China[7] both highlighted the importance of targeted, skills-based female condom promotion in increasing the overall percentage of sex acts protected by either female or male condoms as a result of female condom interventions.
Common Barriers
Relationship norms that govern trust, communication, and decision-making are a key barrier to use of both male condoms and female condoms. Furthermore, product attributes, perceived effect on male sexual pleasure (and, to a lesser extent, female pleasure), male partner and health provider attitudes, and self-efficacy to negotiate female condom use and insert the product are the factors most likely to determine whether women use female condoms. In many studies, female condom use has been low and has generally declined after introductory campaigns due to lack of sustained availability. There is some anecdotal evidence from both developed and developing countries that female condoms are used by men who have sex with men for STI/HIV prevention during anal intercourse. However, no published, peer reviewed evidence yet exists on their safety and efficacy for this purpose. Female condoms are not WHO pre-qualified for use in anal intercourse and, therefore, there is currently no international recommendation that either men or women should use female condoms for STI/HIV prevention during anal sex. For more information on female condoms, please visit the UN Every Woman Every Child initiative webpage.
[1] UNAIDS. (2013). UNAIDS report on the global AIDS epidemic 2013. [2] Technologies, Caucus on New and Underused Reproductive Health. (2012). Female Condom Product Brief (Vol. 2014). [3] Children, United Nations Commission on Life-Saving Commodities for Women and. (2012). Contraceptive Commodities for Women's Health. [4] UFAC. (2013). Female Condom Product Brief. [5] Beksinska, Mags E, Piaggio, Gilda, Smit, Jennifer A, Wu, Junqing, Zhang, Yufeng, Pienaar, Jacqueline, . . . Joanis, Carol. (2013). Performance and safety of the second-generation female condom (FC2) versus the Woman's, the VA worn-of-women, and the Cupid female condoms: a randomised controlled non-inferiority crossover trial. The Lancet Global Health, 1(3), e146-e152. [6] Choi, Kyung-Hee, Hoff, Colleen, Gregorich, Steven E, Grinstead, Olga, Gomez, Cynthia, & Hussey, Wendy. (2008). The efficacy of female condom skills training in HIV risk reduction among women: a randomized controlled trial. American Journal of Public Health, 98(10), 1841. [7] Liao, S, Weeks, MR, Wang, Y, Nie, L, Li, F, Zhou, Y, . . . Li, J. (2011). Inclusion of the female condom in a male condom-only intervention in the sex industry in China: a cross-sectional analysis of pre-and post-intervention surveys in three study sites. Public health, 125(5), 283-292.


 This website is made possible by the support of the American People through the
This website is made possible by the support of the American People through the