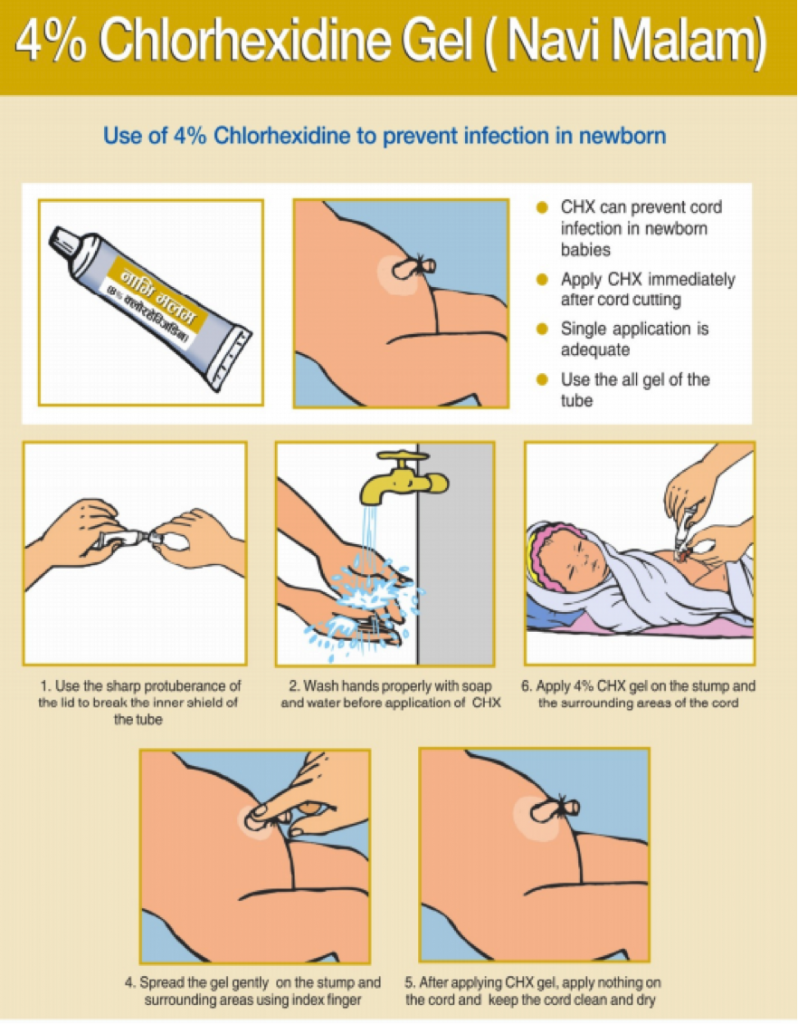
About Chlorhexidine
Few other interventions have as much promise as Chlorhexidine to rapidly reduce newborn deaths at an affordable price—less than $1 per dose.
Globally, neonatal infections are estimated to account for over 1 million newborn deaths annually (over a third of the total burden), with most occurring in sub-Saharan Africa and South Asia. The newly cut umbilical cord is an entry point for bacteria that cause newborn sepsis and death and may also lead to cord infection that can spread to surrounding tissues and the blood stream.
Commodity Context
7.1% chlorhexidine digluconate, a low-cost antiseptic, prevents deadly infections that enter an infant’s body through a newly cut umbilical cord. Few other interventions have as much promise to rapidly reduce newborn deaths at an affordable price—less than $1 per dose[1]. Chlorhexidine has no toxicity risks and virtually no potential for misuse. It has a long shelf life, requires no cold chain, and is extremely easy to apply with minimal training and no equipment. These factors make it suitable for hospital, health center, and home care alike. Few other interventions have demonstrated such potential for rapidly reducing newborn mortality across so many settings for such a low cost.
In July 2013, WHO included 7.1% chlorhexidine digluconate (delivering 4% chlorhexidine) for umbilical cord care on the WHO Model List of Essential Medicines for Children[2]. In October 2013, WHO issued new guidelines for umbilical cord care that recommend daily application of 7.1% chlorhexidine digluconate to the umbilical cord stump for the first week of life in areas with high neonatal mortality[3].
The product is available in a gel and an aqueous solution (liquid). The global Chlorhexidine Working Group’s recommended dose for a single day application is 3g of gel (as currently practiced in Nepal) or 10ml of liquid. For a 7-day application, the recommended dose is 20g of gel and 30ml of liquid. These product sizes allow for wastage and will provide sufficient product for the indicated term of application.
The WHO recommends daily chlorhexidine application to the umbilical cord stump during the first week of life for newborns who are born at home in settings with high neonatal mortality (30 or more neonatal deaths per 1000 live births). In low neonatal mortality settings, clean, dry cord care is recommended for newborns born in health facilities and at home. Use of chlorhexidine in these situations may be considered only to replace application of a harmful traditional substance, such as cow dung, to the cord stump.
Where consumer research has been conducted, mothers have shown a strong latent demand for a purpose-made antiseptic like chlorhexidine. They have demonstrated the ability to use chlorhexidine correctly and have accepted that chlorhexidine makes cord detachment take 1-2 days longer.
Products Available
Chlorhexidine digluconate, in various forms, has been used for nearly 50 years and has applications across a broad range of veterinary, dental, and medical indications. However, 7.1% chlorhexidine digluconate is a novel formulation specifically intended for umbilical cord care and at this time there are a limited number of manufacturers.
The global Chlorhexidine Working Group is committed to establishing a supply of high-quality chlorhexidine for umbilical cord care and is actively engaged in evaluating and providing technical assistance to qualified manufacturers. Currently, Lomus Pharmaceuticals Pvt. Ltd (Nepal) and Drugfield Pharmaceuticals Ltd. (Nigeria) are producing 7.1% chlorhexidine digluconate gel commercially and manufacturing in East Africa and Bangladesh are expected to be underway in 2015. A 7.1% chlorhexidine digluconate aqueous solution (liquid) is also available at through the UNICEF supply catalogue[4].
Product Efficacy
Recent community-level randomized controlled trials in Nepal, Pakistan, and Bangladesh have shown that applying a 7.1% chlorhexidine digluconate (delivering 4% chlorhexidine) product to the umbilical cord saves lives[5; 6; 7]. Across the three countries, data from over 54,000 newborns showed an aggregate 23% reduction in neonatal mortality (not including deaths in the first few hours of life) and a 68% reduction in severe infections for the chlorhexidine intervention groups. These are some of the largest effect sizes seen in any neonatal intervention[8]. It is estimated that chlorhexidine has the potential to reduce overall newborn mortality risk by up to 18%, resulting in over half a million newborn lives saved[8; 9].
Common Barriers
Millions of mothers and health providers around the world continue to have a strong desire to apply something to the umbilical cord stump, and putting nothing on the cord stump is simply unacceptable in some cultures or communities. In the absence of a specifically recommended product, they use a variety of traditional and non-traditional substances, including breast milk, cooking and motor oil, dried cow dung, ash, alcohol, traditional herbs, salty water, mustard seed oil, turmeric, antibacterial ointments, and numerous other preparations.
Chlorhexidine for cord care typically adds 1-2 days to the time it takes the cord stump to fall off. Most women (and families and communities) value speedy detachment of the stump, so communication strategies, messages, and materials should anticipate and address the issue of potential delay.
For more information on chlorhexidine, please visit the UN Every Woman Every Child initiative webpage and the Health Newborn Network.
[1] PATH. (2013). Breakthrough innovations that can save women and children now. from http://www.path.org/innovations2015/
[2] Organization, World Health. (2013). WHO model list of essential medicines for children: 4h list (updated) April 2013.
[3] WHO. (2013). WHO recommendations on postnatal care of the mother and newborn.
[4] Network, Healthy Newborn. (2014). Chlorhexidine for umbilical cord care.
[5] Mullany, Luke C, Darmstadt, Gary L, Khatry, Subarna K, Katz, Joanne, LeClerq, Steven C, Shrestha, Shardaram, . . . Tielsch, James M. (2006). Topical applications of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in southern Nepal: a community-based, cluster-randomised trial. The Lancet, 367(9514), 910-918.
[6] Soofi, Sajid, Cousens, Simon, Imdad, Aamer, Bhutto, Naveed, Ali, Nabeela, & Bhutta, Zulfiqar A. (2012). Topical application of chlorhexidine to neonatal umbilical cords for prevention of omphalitis and neonatal mortality in a rural district of Pakistan: a community-based, cluster-randomised trial. The Lancet, 379(9820), 1029-1036.
[7] Arifeen, Shams El, Mullany, Luke C, Shah, Rasheduzzaman, Mannan, Ishtiaq, Rahman, Syed M, Talukder, M Radwanur R, . . . Santosham, Mathuram. (2012). The effect of cord cleansing with chlorhexidine on neonatal mortality in rural Bangladesh: a community-based, cluster-randomised trial. The Lancet, 379(9820), 1022-1028.
[8] Children, UN Commission on Life Saving Commodities for Women and. (2012). Comissioner's Report.
[9] Hodgins, Steve, Pradhan, YV, Khanal, Leela, Upreti, Shyam, & KC, Naresh Pratap. (2013). Chlorhexidine for umbilical cord care: game-changer for newborn survival? Global Health: Science and Practice, 1(1), 5-10.